KJ/P∆VIS – Objects of introspection — inseparable, intrinsic, identity. A collection of works by Katherine Jane Pavis
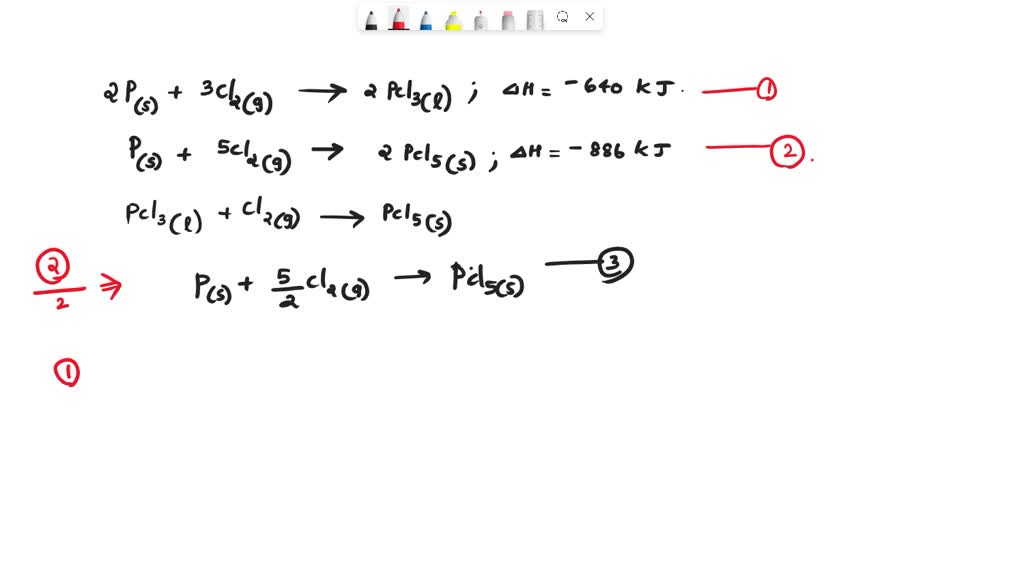
SOLVED: Find the ΔH (in kJ) for the reaction: PCl3(l) + Cl2(g) → PCl5(s) Given: 2 P (s) + 3 Cl2(g) → 2 PCl3(l) ΔH = -640 kJ2 P (s) + 5
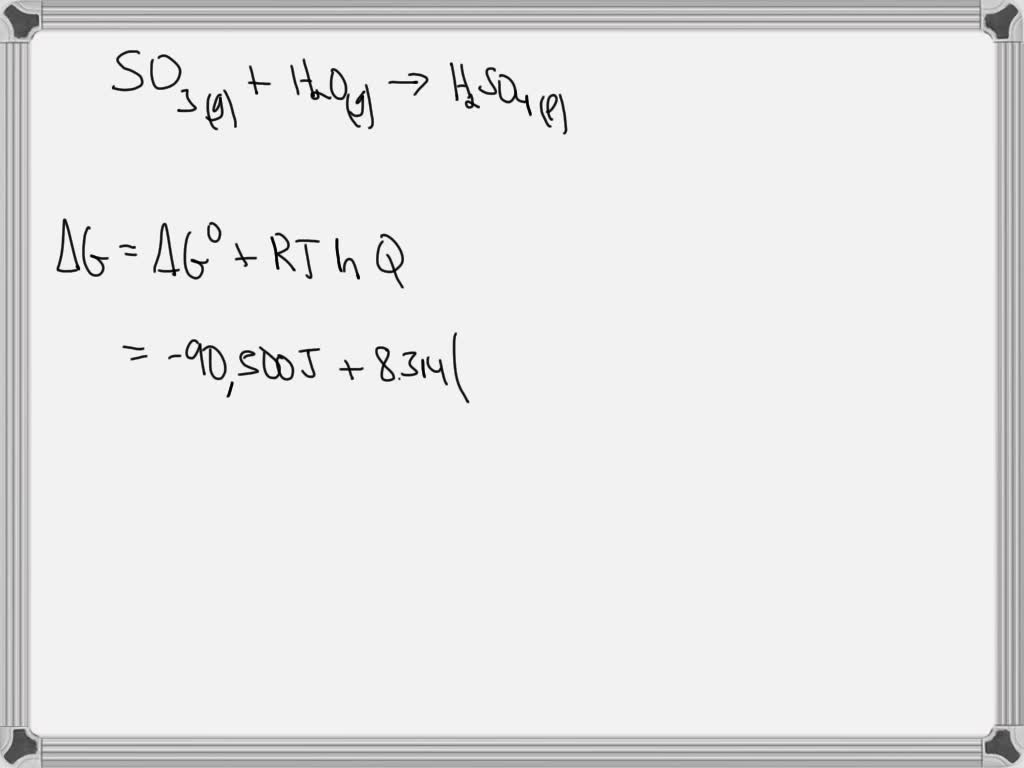
SOLVED: Calculate ΔGrxn (in kJ) at 298 K under the conditions shown below for the following reaction. SO3 (g) + H2O (g) → H2SO4 (l) ΔG°= -90.5 kJ P(SO3) = 0.20 atm, P(H2O) = 0.88 atm

KJ WORKS CZ 75 P-09 Tactical GBB Pistol (GAS, TAN) MPN: P-09-TAC-TAN $102.00 - IceFoxes.com Products

Evaluate the work in KJ for a two step process consisting of an expansion with n=1.0 from P 1 =3 bar, V 1 = 0.1 m 3 to V = 0.15 m

Calculate the standard enthalpy of the reactions 2C (graphite) + 3 H2(g)→ C2H6(g) from the following Δ H^∘ value (i) C2 H6(g) + 72 O2 (g)→ 2 CO2(g) + 3 H2 O (